Maple Syrup Urine Disease (MSUD)
Disclaimer
Metabolic crises in infants and children with organic acid disorders are complex medical emergencies and must be treated as such to avoid death or serious brain injury. This protocol is only a guideline and should NOT be used for definitive treatment without metabolic consultation. It is essential to call or page the on-call genetics/metabolism fellow, or failing this, the on-call metabolic attending at your hospital or nearest pediatric tertiary care center, as rapidly as possible. Please read our Terms of Use.
Click here to download a PDF of the MSUD Acute Illness Materials.
ABOUT:
The acute illness materials are meant as a guideline for healthcare professionals treating the sick infant or child who is known to have maple syrup urine disease (MSUD). These materials were developed at Boston Children’s Hospital under the direction of Dr. Harvey Levy, Senior Physician in Medicine/Genetics and Dr. Jonathan Picker, Fragile X Program Director, and were most recently updated in 2020.
Introduction:
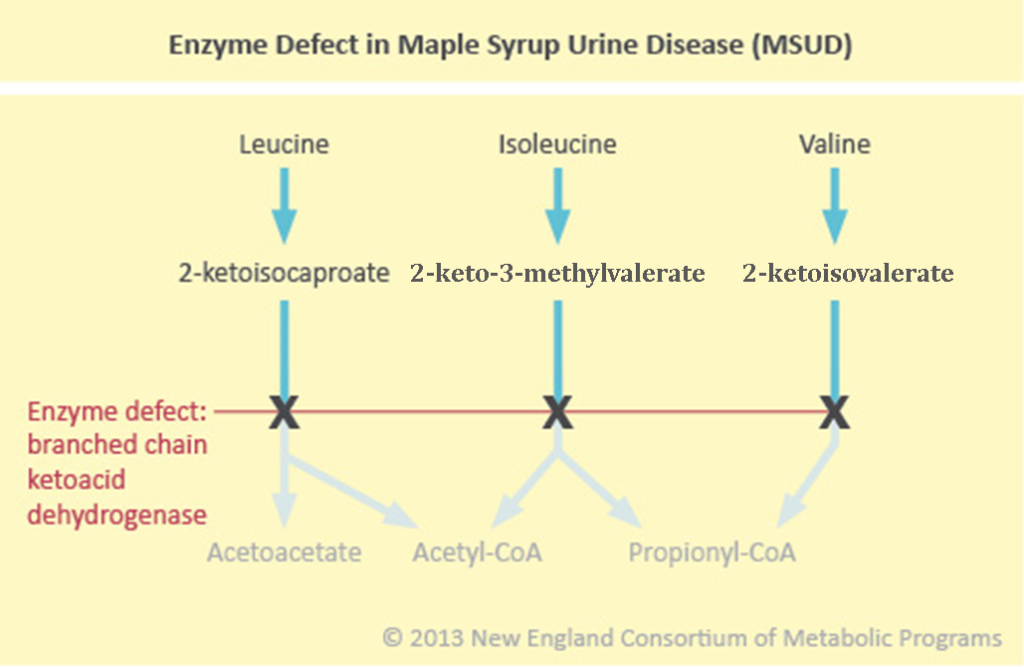
Maple Syrup Urine Disease (MSUD) is an autosomal recessive organic acid/amino acid disorder due to a defect in the Branched Chain Ketoacid Dehydrogenase (BCKD) enzyme, which catalyzes the breakdown of branched chain ketoacids. These ketoacids form from the breakdown of the branched chain amino acids (BCAA): leucine, isoleucine, and valine. As a consequence, the ketoacids and their precursor BCAA accumulate in blood with the toxic metabolic components being leucine and the ketoacids.
Pathophysiology:
The types of presentations of MSUD include the classic (most severe and most common form at risk for major metabolic crisis in newborn and later periods), intermediate (elevations in metabolites with little or no risk for metabolic crisis), and intermittent (asymptomatic but at risk for metabolic crises during acute illness, usually infection).
Catabolic stress such as normal perinatal catabolism or an acute illness (e.g. infection, injury, surgery, febrile illness) produces endogenous protein breakdown leading to increase in the BCAA and related branched chain ketoacids. When excessive protein is ingested, a similar increase in available amino acids occurs.
Metabolic consequences include:
Increased ketoacids -> ketosis, metabolic acidosis
Increased glucose utilization -> ketosis
Increased leucine -> brain toxicity
Acute Presentation:
Lethargy, irritability
Poor feeding
Nausea, vomiting
Hypotonia, hypertonia, dystonia
Ataxia
Seizures
Coma
Maple syrup odor (urine, ear wax)
Laboratory findings:
Metabolic acidosis with anion gap
Ketosis, ketonuria
Increased BCAA (plasma amino acid analysis)
Parents of children with diagnosed metabolic disorders know the signs of decompensation in THEIR children.
It is important to listen to the parents’ insight into their child’s illness.
Immediate Assessment:
Dextrose stick for blood glucose
Vital signs, cardiovascular stability
Hydration status
Presence of fever; signs of infection
Neurologic status; evidence of increased intracranial pressure, “leucine encephalopathy”*
CT or MRI should be immediately performed on any patient who is encephalopathic
Labs - Blood:
Venous blood gas for blood pH
Electrolytes (low sodium can be a sign of elevated/increasing leucine), measured CO2, glucose
Renal function (BUN, creatinine)
Plasma amino acids (high leucine, isoleucine, valine; elevated levels of allo-isoleucine in blood is pathognomonic of MSUD) - STAT
CBC, differential WBC count, platelet count
Blood culture (if indicated)
Serum amylase, lipase
Labs - Urine:
Urinalysis for specific gravity and ketones
Urine for organic acids
Urine culture (if indicated)
*NOTE: Increased concentrations of leucine are toxic to the brain and accumulations may result in cerebral edema. Caution should be exercised when considering the need for a lumbar puncture.
Management
We recognize that many who access this protocol will not be at a metabolic center and, therefore, not have access to the specific MSUD products required for therapy nor the availability of an immediate amino acid analysis or hemodialysis described below. Should this be the case, we recommend that a patient with MSUD with signs of cerebral edema be stabilized as described below with rehydration and immediately transported to the nearest metabolic center.
Specific management guidelines are listed here, with details below:
Eliminate leucine by stopping intake of all natural protein
Provide hydration and high caloric supplementation
Correct metabolic abnormalities
Monitor/treat cerebral edema
Other considerations during metabolic crises
Treat precipitating factor(s)
Cofactor supplementation
1. Protein intake
All natural protein intake (e.g., breastfeeding, infant formulas) should be halted in the setting of a metabolic crisis. A specialized BCAA-free MSUD formula* should be started as soon as possible; this is key to lowering leucine levels (see “hydration/caloric supplementation” category below). This BCAA-free MSUD formula should be administered until the leucine levels approach targets (leucine: 200 µmol/L for infants and children ≤5 years of age and 300 µmol/L for those >5 years of age). If the patient is unable to take a MSUD formula orally or by nasogastric tube, consider a specialized branched-chain amino acid-free parenteral solution available through specific pharmacies**.
Once target leucine levels are achieved (see above), natural protein should be slowly reintroduced.
*BCAA-free MSUD formulas available in the United States (TABLE #7: Classification of Medical Foods for MSUD)
**Integrity Compounding Pharmacy. Inclusion does not represent endorsement.
2. Hydration/caloric supplementation
All patients with MSUD in metabolic crisis should receive high caloric supplementation to achieve an anabolic state (125-150% of typical energy needs). Catabolism, precipitated by any stressor, can contribute to underlying metabolic decompensation and promote worsening metabolic acidosis and ketosis.
The preferred calorie source is BCAA-free MSUD formula administered enterally, either oral or via nasogastric tube. BCAA-free MSUD formula should generally provide 2 to 3.5 g protein equivalent/kg/day. If BCAA-free MSUD formula is not available or not tolerated, intravenous fluids should be used. Intravenous fluids should be used cautiously in MSUD patients during a metabolic crisis, as high rate intravenous fluids may contribute to the development of brain edema. Hypotonic fluids should NOT be used.
In a metabolic crisis, a patient with MSUD may require intravenous fluids (either peripherally or via a central venous catheter) for rehydration purposes and also for provision of calories. High dextrose-containing fluids (10% glucose) should be administered with the addition of electrolytes (half or full normal saline, and also potassium if urine output is adequate and renal function is sufficient) at the maintenance rate. An intravenous lipid infusion (e.g. intralipid) should be considered to provide increased calories. If leucine levels are not in a range associated with brain edema, it is generally acceptable to use high rate intravenous fluids (1.5 times maintenance rate). Intravenous fluids should be continued until oral fluids are tolerated.
3. Correct metabolic abnormalities
a. Metabolic acidosis/ketosis: this should slowly correct with rehydration and high caloric intake; if serum bicarbonate is below 10 meq/L and blood pH < 7.2, give IV bolus NaHCO3 as 2.5 meq/kg over 30 minutes, then 2.5 meq/kg/day until serum bicarbonate is 24-28 meq/L.
Aims are:
Serum bicarbonate level over 24 meq/L
Absence of ketones in urine
b. Maintain serum sodium at 140-145 meq/L. Monitor urine sodium output to establish loss and replacement requirement. As serum sodium approaches 140-145 meq/L, reduce IV fluids to D10/ 1/2 normal saline and monitor serum sodium closely (hyponatremia enhances brain edema). After 24 hours, adjust sodium intake to provide 4 meq/kg/day. Too much sodium will complicate fluid management.
c. Measure plasma amino acids every 24 hours. The goals for BCAA levels during an acute crisis should be:
Leucine: <200 µmol/L for infants and children ≤5 years of age and <300 µmol/L for those >5 years of age
Isoleucine: 200-400 umol/L*
Valine: 200-400 umol/L*
*Isoleucine and Valine Supplements: It is important to realize that isoleucine and valine levels may drop rapidly and low levels (isoleucine <200 umol/L and valine <200 umol/L) will prevent leucine from dropping by limiting protein synthesis. Low isoleucine and valine also increase the blood:brain barrier transport of leucine due to less inhibition by isoleucine and valine; increased brain leucine produces or enhances brain edema. Add isoleucine and valine at 20-120 mg/kg/day to achieve the target levels above.
d. If blood glucose rises > 200 mg/dL with IV dextrose infusion, begin insulin infusion at 0.05-0.1 unit/kg/hr until blood glucose is controlled.
4. cerebral edema
If neurological signs develop or worsen (vomiting, lethargy, hyperreflexia, clonus), suspect cerebral edema. Critical edema most often occurs during IV therapy, either due to low serum sodium (below 135 meq/L) or continued ketosis and vomiting. Brain edema (with associated brain stem herniation) is the most frequent cause of death in MSUD.
If cerebral edema is suspected, obtain brain CT or MRI. If cerebral edema is confirmed:
Order and start hemodialysis ASAP.
Infuse mannitol at 1- 2 grams/kg over 30-40 minutes.
Add IV lasix for diuresis, but carefully monitor serum sodium to maintain concentration in the 140-145 meq/L range. One can infuse hypertonic saline to maintain sodium in this range.
Hemodialysis: Hemodialysis should be a last resort but may be lifesaving in a patient who presents with coma and seizures and in whom IV therapy may not correct the profound metabolic derangements in time to prevent death from cerebral edema with brain stem compression. This mode of intervention should be instituted in consultation with a pediatric nephrology service.
5. Other Clinical Considerations During an Acute Crisis
a. Vomiting is the nemesis of MSUD. It provokes and/or exacerbates ketoacidosis and complicates enteral therapy. Zofran at 2-4 mg every 6-8 hours can be effective in controlling vomiting.
b. Acute pancreatitis: This can sometimes accompany acute metabolic episodes. Monitor serum amylase and lipase.
6. Precipitating factors
Acute metabolic decompensation in a patient with MSUD is almost always precipitated by a stressor, such as infection, injury, surgery, hormonal changes, or significant dietary changes (involving increased natural protein intake). It is of utmost importance to identify and address the precipitating factor for the patient’s metabolic decompensation as treatment of the stressor will facilitate treatment of the metabolic derangements.
Infection: Antibiotics should be provided to treat the particular infection.
Surgery: Prevention of metabolic decompensation as a result of the stress of surgery requires intravenous fluids with D10 prior to and after surgery until oral fluids are tolerated, avoiding prolonged fasting to the extent possible, addressing pain issues, and providing adequate calories to promote fast healing.
Hormonal Changes: The specific situation needs to be assessed and possible dietary changes should be undertaken in accordance with the patient’s hormonal status (e.g., puberty, growth spurt, menarche, thyroid disorder).
Change in Diet: A change in the patient’s diet, with excessive natural protein intake, should be assessed. This would be easily addressed by adjusting the natural protein intake.
7. Cofactor supplementation
Some patients with MSUD are responsive to thiamine supplementation (in the long-term, not during an acute metabolic episode). These patients are more likely to have intermediate rather than classic MSUD with persistent elevations of MSUD metabolites but no major metabolic crises.
Monitoring the Patient
Clinical parameters:
Mental status
Hydration status/fluid balance/oral intake
Evidence of bleeding (if thrombocytopenic)
Symptoms of infection (if neutropenic)
Monitor for signs/symptoms of renal failure
Biochemical parameters:
Electrolytes, measured CO2, glucose, blood gases (q 4-6 hours or as indicated)
CBC with differential, platelets (if needed)
Renal function (if needed)
Urine for ketones and specific gravity with every void
Plasma amino acids (once daily)
Recovery:
The patient should be kept NPO until his/her mental status is improved. Anorexia and nausea/vomiting during the acute crisis make a significant oral intake unlikely and can exacerbate the ketoacidosis. Once the patient is stable and accepting enteral feeding, the plasma amino acids should ideally be monitored daily to reestablish amino acid homeostasis. On the basis of these levels, the BCAA-free MSUD formula with added source of natural protein and low protein foods are adjusted to aim for plasma levels as follows:
Leucine: 75-200 µmol/L for infants and children ≤5 years of age and 75-300 µmol/L for those >5 years of age
Isoleucine: 200-400 µmol/L
Valine: 200-400 µmol/L
Other amino acids: Within the normal range
This will require careful attention to the amount of BCAA-free MSUD formula ingested, the amount of natural protein added to the MSUD formula, the amount of low protein foods ingested and the amount of supplemental isoleucine and valine added to the MSUD formula (each supplement should be available in the pharmacy as a 100 mg/10 ml solution). Dietary treatment should only be done by or with the guidance of a metabolic physician and nutritionist.
References:
Frazier DM, Allgeier C, Homer C, et al. Nutrition management guideline for maple syrup urine disease: an evidence- and consensus-based approach. Mol Genet Metab. 2014;112(3):210-217. doi:10.1016/j.ymgme.2014.05.006
Southeast Newborn Screening and Genetics Collaborative (SERC) and Genetic and Metabolic Dietitians International (GMDI). MSUD Nutrition Management Guidelines and Toolkit.
Rodan LH, Aldubayan SH, Berry GT, Levy HL. Acute Illness Protocol for Maple Syrup Urine Disease. Pediatr Emerg Care. 2018;34(1):64-67. doi:10.1097/PEC.0000000000001299
Disclaimer
Metabolic crises in infants and children with organic acid disorders are complex medical emergencies and must be treated as such to avoid death or serious brain injury. This protocol is only a guideline and should not be used for definitive treatment without metabolic consultation. It is essential to call or page the on-call genetics/metabolism fellow, or failing this, the on-call metabolic attending at your hospital or nearest pediatric tertiary care center, as rapidly as possible. Please read our Terms of Use.